Radioactivity
In 1899 Ernest Rutherford showed the existence of alpha and beta radiation. Paul Villard discovered gamma radiation a year later.
Alpha Radiation
α particles are the nuclei of the helium atom.
When an element undergoes alpha decay , a helium atom is removed – so the original element loses two protons and two neutrons and is transformed into a lighter element.
![]()
Example
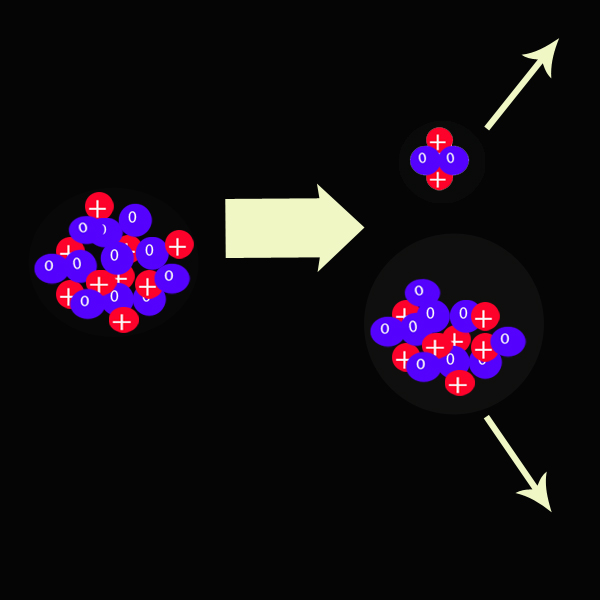
The radioisotope Platinum 190 decays into Osmium 186 and a Helium particle.
![]()
Alpha particles can be stopped by a thin sheet of paper.
Beta Radiation
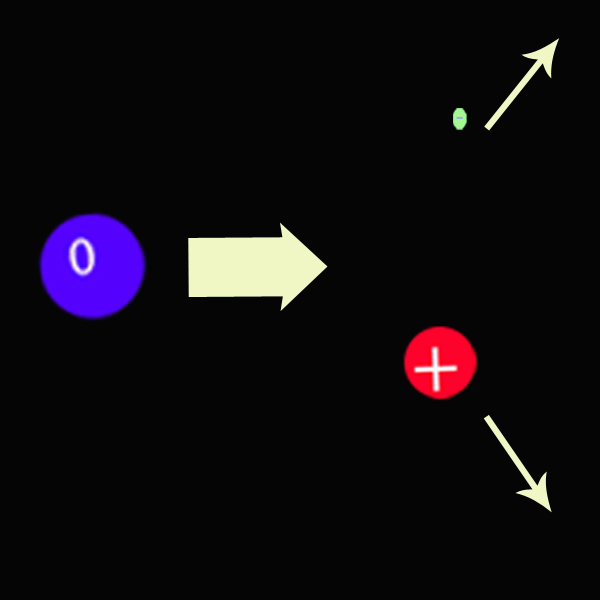
β particles are electrons that have been emitted from the atom. Free neutrons are radioactive with a half-life of around 11 minutes. A neutron converts into a proton and electron.
![]()
As a result, the original element gains a proton, so will be increase its atomic number by 1 , but keep its atomic mass since the mass of the neutron is the same as the proton and the mass of the electron is negligble.
![]()
Example
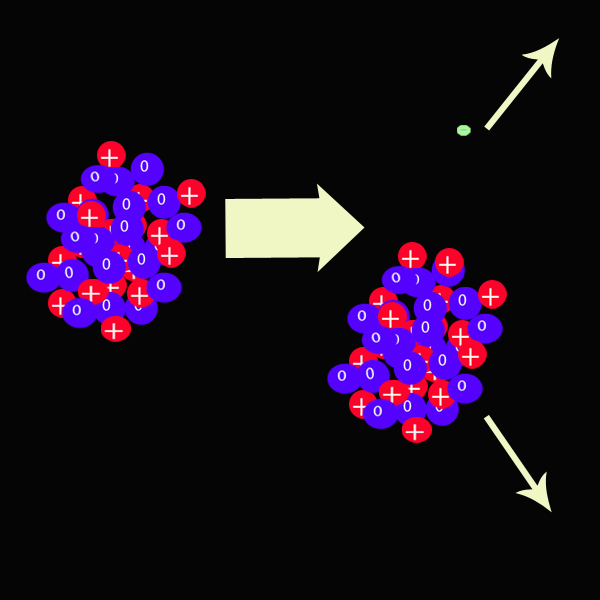
Strontium undergoes beta decay and turns into Yttrium.
![]()
Beta particles can be stopped by a few cm of aluminium.
Gamma Radiation
γ decay is the result of energy state changes within the nucleus, often following α or β decay where the newly formed nucleus is not in its lowest energy state.
The excess energy is emitted as radiation – light waves with very high frequency and very short wavelength ( approx. 1 x 10 -12 mm )
Radioactive Decay and Half-life
The Royal Society of Chemistry Interactive Periodic Table
Libre Text books Nuclear Radioactivity
