Early Greek atomic model
The early Greeks believed that all matter was made up of tiny uncuttable building blocks. Democritus of Abdera, a disciple of Leucippus of Miletus , named these building blocks of matter atomos around 430 bce. He suggested that atoms of the four different elements earth, air, fire, and water are different sized spheres which move around in space.




Changes to the matter of a material occoured when these atoms collided, rebound or stuck together.
Dalton’s atomic model
In the early 1800's John Dalton used experimental results to propose a new model of the atom in which he suggested the following:
- All matter consists of extremely small particles called atoms.
- Atoms are indestructible and resist changes.
- Atoms could not be created, destroyed, cut up or transformed into atoms of other elements.
- Elements are defined by the mass of their atoms.
- All atoms of an element are identical in shape, size, and mass.
- When atoms are involved in chemical reactions, they combine in small whole-number ratios to form molecules.
- Two types of atoms could form molecules of different whole-number ratios.
He used the law of conservation of massin the late 1700s as the basis for these conclusions.

In the years after Dalton described his atomic model, multiple experiments were performed that proved that charged particles exist.
In 1833 Michael Farady studied electrolytic solutions and realised two requirements must be met for matter to conduct electricity:
- The matter must be composed of, or contain, electrically charged particles.
- These particles must be mobile - free to move under the influence of an external applied electric field.
Faraday called the charge carrier ions, after the ancient Greek word "to go".
An ion is an atom molecule with a net electrical charge - its total number of electrons is unequal to its total number of protons.
Ions move towards the electrode of the opposite charge :
A cation is a positively charged ion with fewer electrons than protons. The name comes from ancient Greek for "going down".
An anion is a negatively charged ion with more electrons than protons.The name comes from ancient Greek for "going up".
Svante Arrhenius investigated the galvanic conductivity of electrolytes.
His work on electrolytic dissociation won him the 1903 Nobel Prize in Chemistry.
Thomson's atomic model
In 1897 J.J. Thomson discovered a negatively charged particle, which he called the electron . Thomson thought that the electrons must be inside the atoms of elements.
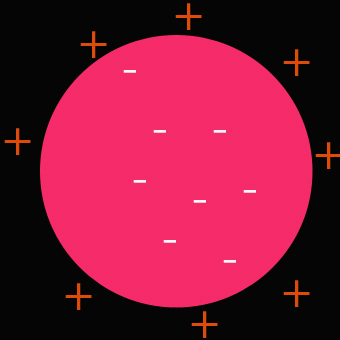
This supported the “plum-pudding” atomic model first proposed by William Thomson ( Lord Kelvin ) .
In 1899 Ernest Rutherford showed the existence of alpha and beta radiation.
Rutherford Scattering Simulation
In 1902 he and Frederick Soddy theorised that elements could disintegrate and be transformed into other elements.
Ernest Rutherford won the 1908 Nobel Prize in Chemistry, Frederick Soddy won the 1921 Nobel Prize in Chemistry
.
Quantum atomic model
The idea that all forms of energy are released in discrete units or bundles called quanta and that radiation consists of quanta with specific energies determined by a new fundamental constant was suggested by Max Planck around 1900 and won him the 1918 Nobel Prize in Physics.
Planck’s constant h relates the energy E of a photon to the frequency of its corresponding electromagnetic wave f
E = hf
The equation for wavelength (λ) is λ = v/f , where v is the velocity of the wave. Since a photon is travelling at the speed of light ( c ) , the equation for wavelength is λ = c/f , so f = c /λ
Substituting for frequency, the equation becomes
E = hc / λ
In 1905 Albert Einstein used the concept of Planck’s constant to explain the photoelectric effect in 1905. He showed that light could be thought of as a stream of photons, each with energy E=hf.
His work won him the 1921 Nobel Prize in Physics.
Robert Milikan determined the charge of a single electron with his
His work on this, and the photoelectric effect, won him the 1923 Nobel Prize in Physics.
Rutherford's atomic model
In 1911 Ernest Rutherford carried out experiments with gold-foil,from which he deduced that the atom has a tiny, positively charged massive core called the nucleus, around which negatively charged electrons orbit.
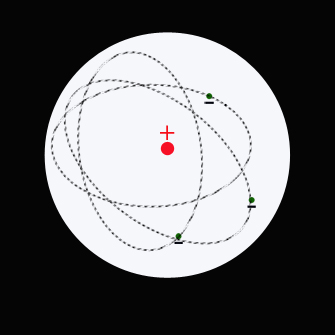
The empty space between the nucleus and the electrons takes up most of the volume of the atom.
The motion of the electrons in the Rutherford model was unstable because, according to classical mechanics and electromagnetic theory, any charged particle moving on a curved path emits electromagnetic radiation; thus, the electrons would lose energy and spiral into the nucleus.
In June 1912 John William Nicholson created an atomic model that quantized angular momentum as h/2π. He also created a nuclear and quantum theory that explains spectral line radiation as electrons descend toward the nucleus, identifying unknown solar and nebular spectral lines.
In 1912 Henry Moseley created the radium battery , the world's first atomic battery. In 1913 he discovered that an element’s atomic number is identical to the number of protons it has. This led to the realization the periodic table should be ordered by atomic number (called Z )- and not atomic mass ( called A)
Bohr's atomic model
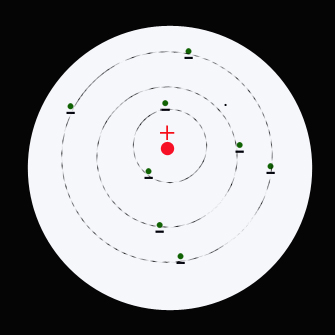
In 1913 Niels Bohr modified the Rutherford model and proposed a quantized shell model of the atom requiring that the electrons move in orbits - paths around the nucleus of an atom of fixed size and energy:
- Each orbit has a different energy level associated with it.
- The energy of an electron depends on the size of the orbit and is lower for smaller orbits.
- The atom will be completely stable in the state with the smallest orbit.
- Radiation can occur only when the electron jumps from one orbit to another.
- Energy can be absorbed by electrons to move from a lower energy orbit to a higher energy orbit.
- Energy can be released by electrons to move from a higher energy orbit to a lower energy orbit. A photon with enegy E =hf is emitted .
An atomic orbital is a region in an atom where an electron is most likely to be found.
Atom Quantum Numbers
The Principal Quantum Number ( n ) (Shell number)
This describes the energy of an electron and the most probable distance of the electron from the nucleus. In other words, it refers to the size of the orbital and the energy level an electron is placed in.
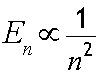
The principal quantum number, n, has lowest energy state n = 1 , first excited state n = 2 , and so on - where n is a natural number.
The first shell contains a maximum of 2 electrons. The second shell contains a maximum of 8 electrons. The series is 2, 8 , 8 , 18, 18, 32
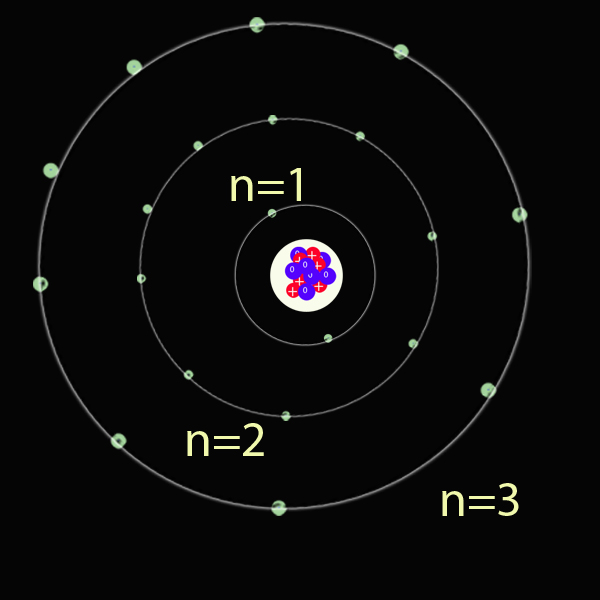
This impacts the periodic table
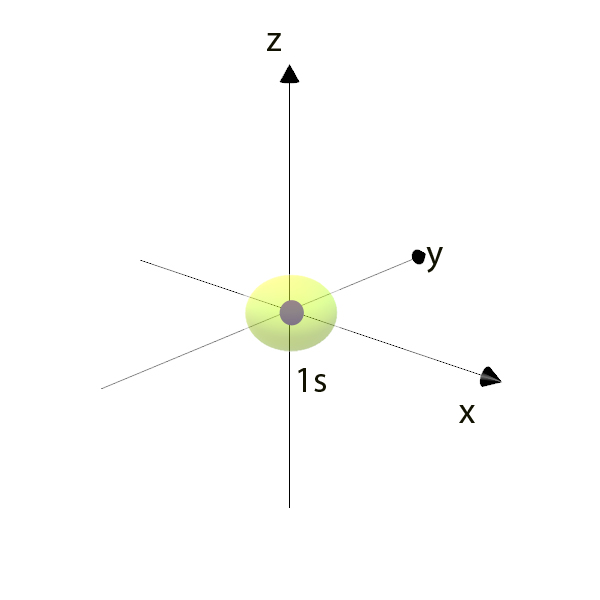
The Orbital Angular Momentum Quantum Number ( l )
Aka Azimuthal quantum number, this describes the shape of the orbital.
l = 0 is the s orbital. (Spherical)
l = 1 is the p orbital.
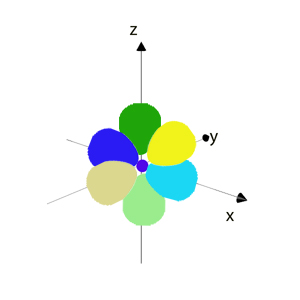
The p orbitals are shaped like dumbells, orientated along the x, y, and z axes.
l = 2 is the d orbital.

The d orbitals consist of five different shapes known as dxy, dyz, dzx, dz², and dx²-y².
l = 3 is the f orbital.
The Magnetic Quantum Number ( m l )
This describes the energy levels in a subshell and determines the number of orbitals and their orientation within a subshell.
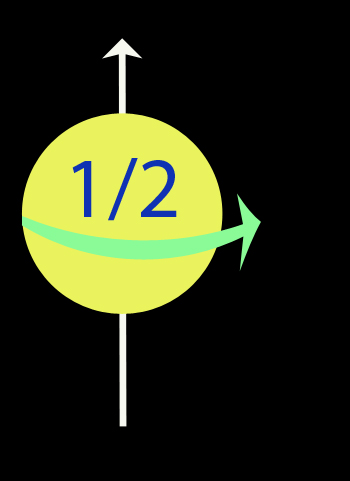
The Electron Spin Quantum Number ( ms )
refers to the spin on the electron, which can either be up or down
Spin
Spin Up - anticlockwise, top towards north.
Spin down - clockwise, top towards south.
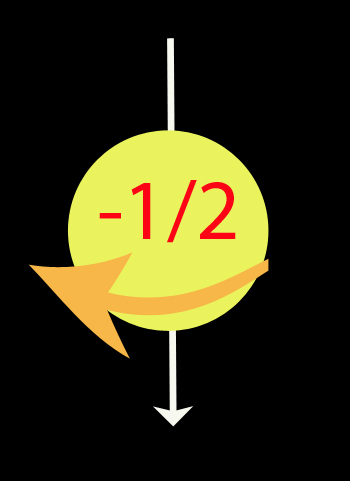
His work won him the 1922 Nobel Prize in Physics.
In 1923 Arthur Compton confirmed that electromagnetic radiation could also be described as photon particles.
His work The Compton Effect won him the 1927 Nobel Prize in Physics.
In 1925 Wolfgang Pauli proposed that no two electrons in an atom could have identical sets of quantum numbers .
His Exclusion Principlework won him the 1945 Nobel Prize in Physics.
Louis de Broglie suggested that the wave-particle duality of light extends to matter in his postulate called the de Broglie wavelength
His work won him the 1929 Nobel Prize in Physics.
In 1926 Erwin Schrödinger used mathematical equations to describe the probability of finding electrons in specific positions .The Schrödinger Equation
These equations no longer state with certainty where electrons can be found but instead describe the region of space where it is highly probable that an electron could be found. His famous thought experiment is called the cat paradox, aka Schrödinger's Cat
His work won him the 1933 Nobel Prize in Physics, which he shared with Paul Dirac , who predicted the existence of antiparticles.
In 1927 Werner Heisenberg formulated his Uncertainty Principle , which stated that both the position and speed of a particle, such as a photon or electron, cannot be known with perfect accuracy.His work won him the 1932 Nobel Prize in Physics
In 1932 James Chadwick discovered a neutral particle with about the same mass as a proton located inside the nucleus of the atom - which he called the neutron. His work won him the 1935 Nobel Prize in Physics.
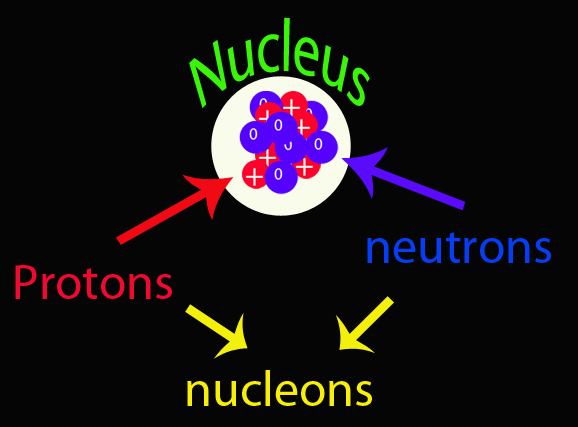
A nucleon is inside the nucleus of the atom and is either a proton or neutron.
In 1935 Hideki Yukawa used the Heisenberg Uncertainty Principle to predict the existance of a short lived particle which is exchanged between nucleons as the carrier of forces .
His work won him the 1949 Nobel Prize in Physics.
In 1936 , Carl D Anderson and Seth Neddermeyer discovered the muon.
Carl Anderson also discovered the antimatter electron , which he called the positron. For this he was awarded the 1936 Nobel Prize in Physics.
The Standard Model
In 1964 Murray Gell-Mann proposed that protons and neutrons consist of elementary particles which he called quarks, named ,
"Three quarks for Muster Mark!" the novel Finnegans Wake, by James Joyce
quarks.
His work won him the 1969 Nobel Prize in Physics
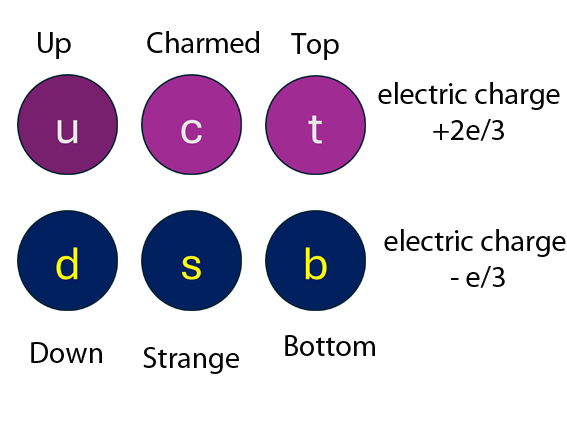
Originally, there were three quarks : Up, Down and Strange.
Now, there are six flavours of quark : Up,Down,Charmed, Strange,Top and Bottom. The three colours of Quark - Red, Green and Blue - have nothing to do with visible colour, but are labels for spin state. This is to satisfy Pauli's Exclusion Principle, since quarks are Fermions.
Up quarks have charge 2/3 of an electron , Down and Strange quarks both have charge - 1/3 of an electron. They are stuck together with gluons.
The Standard Model All about quarks
The six quark flavours :
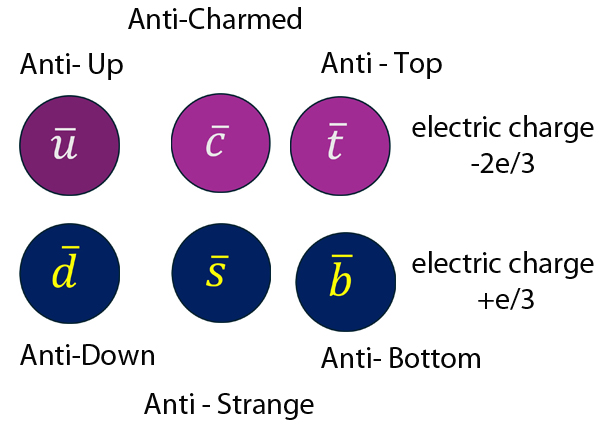
Anti-matter has anti-quarks.
Baryons
Three quark combinations are called baryons.
A proton has two up and one down quarks held together by gluons - massless carriers of the strong force:
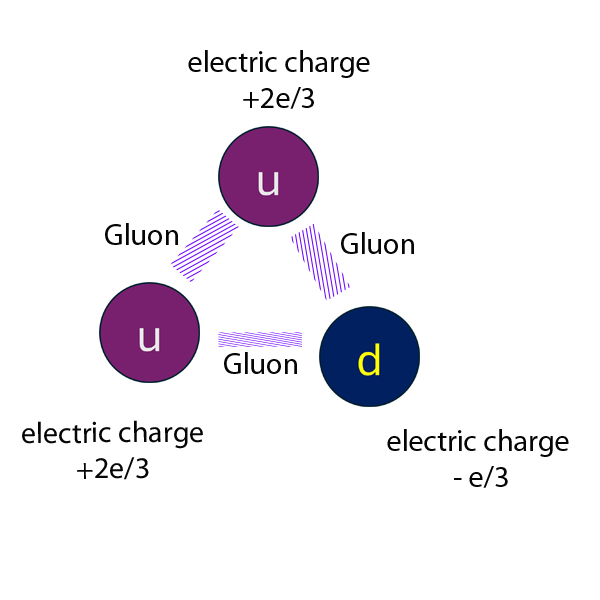
Electric charge 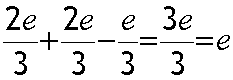
A Neutron has two down and one up quarks :
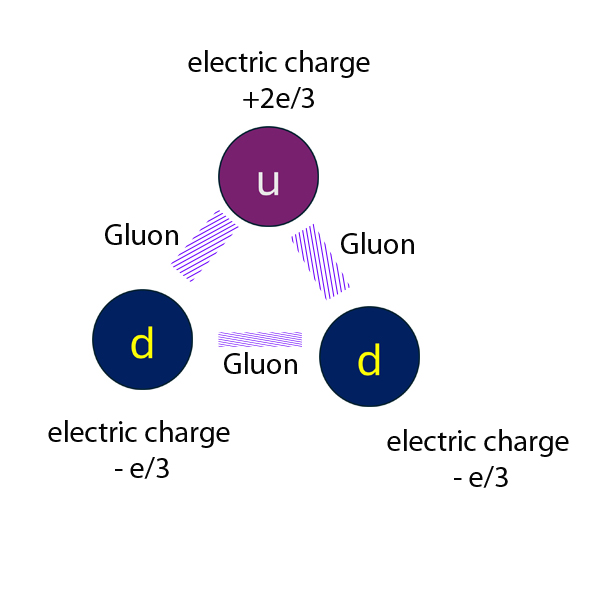
Electric charge 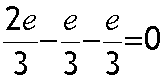
There are many more baryons.
Anti -Baryons
The anti-matter version. The charges are opposite.
Anti-proton :
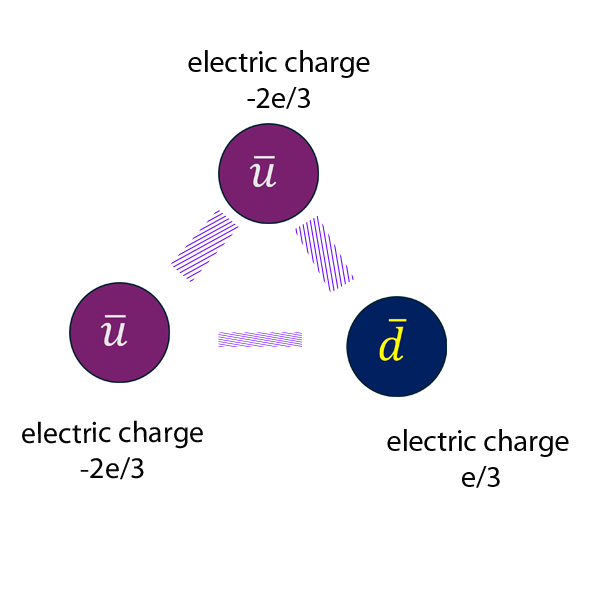
Electric charge 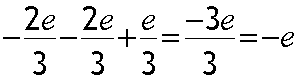
Anti- neutron
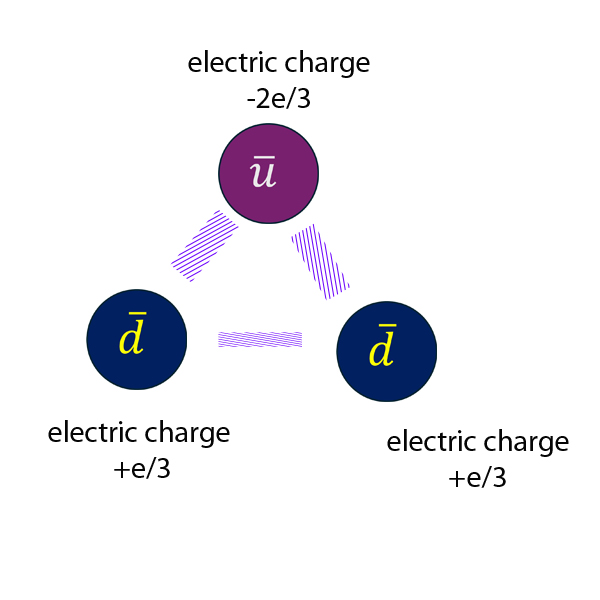
Electric charge 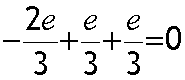
Again, there are many more combinations.
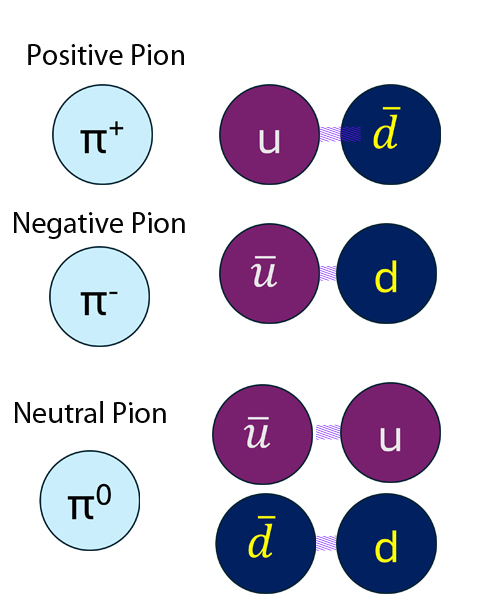
Mesons
Mesons are formed by two quarks in a quark-antiquark pair. again, the quarks are stuck together with gluons.
Mesons are bosons, since they have integer spin.
Pions are an example :
Fermion or Boson
Fermions :
- Named after Fermi
- Make up matter
- Take up space
- Have half-integer spin
- Are constrained by the Pauli exclusion principle.
- The wavefunction for a collection of fermions is antisymmetric
Fermions include electrons, protons, neutrons.
Bosons :
- Named after Bose
- Carry forces
- Pile up on each other
- energy distribution is described by Bose-Einstein statistics.
- Have integer spin or no spin.
- Are not constrained by the Pauli exclusion principle.
- The wavefunction for a collection of bosons is symmetric.
Bosons include photons, alpha particles and helium atoms.
Mesons are bosons
Four Fundamental Forces
- Gravitational - acts on all particles.
Effects very weak on sub-atomic particles. - Electromagnetic - acts on all electrically charged particles.
- Strong - binds nucleons together.
Does not work on all particles - Weak - involved in beta decay.
Works on all particles.
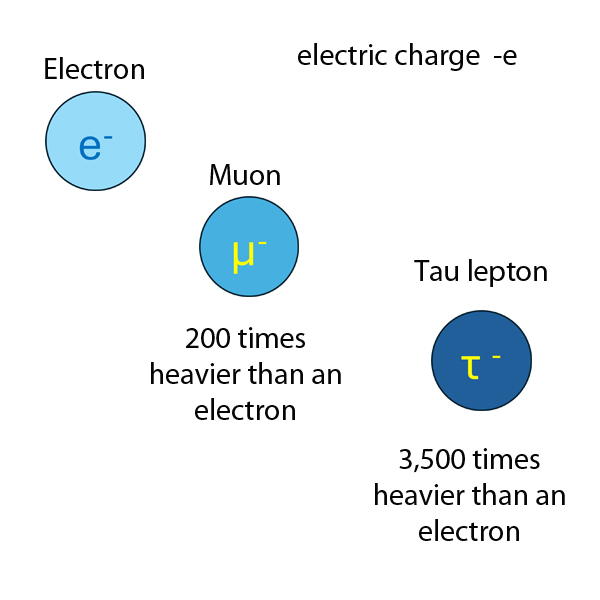
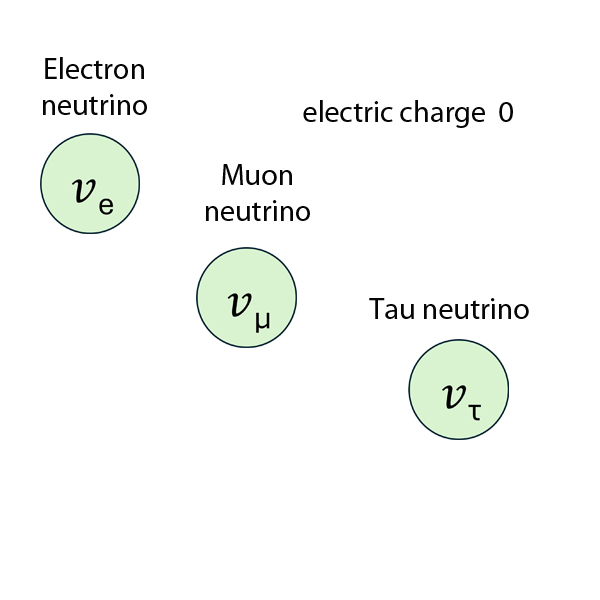
Hadron or Lepton
Hadrons
Particles affected by the Strong Force are called hadrons.
Hadrons are viewed as being composed of quarks, either as quark-antiquark pairs (mesons) or as three quarks (baryons).
Leptons
The word lepton comes from the Greek leptos, meaning ‘thin’ or ‘lightweight’. Leptons are fundamental particles that don't feel the effects of the strong nuclear force.